Sterically Hindered Unnatural α–Amino Acid Synthesis
α,α–Disubstituted–α–amino acids, which are unnatural α–amino acids, are the important partial structure of pharmaceuticals and organocatalysts. Therefore, efficient synthesis methods are important issues in pharmaceutical and synthetic chemistry. The reaction using azlactone, an α–amino acid equivalent having higher acidic α–proton, is widely used in chemical synthesis for unnatural α–amino acids. However, azlactone has generally been used as a nucleophilic agent in cross-coupling reactions with electrophilic agents. On the other hand, it was an underdeveloped area for use in reactions with nucleophiles.
Therefore, we have been developing iron-catalyzed dehydrogenative cross-coupling reactions using the azlactone dimer strategy. Cross-coupling reactions with nucleophiles were achieved using radical and cation intermediate from the homo or hetero cleavage of the azlactone dimer.
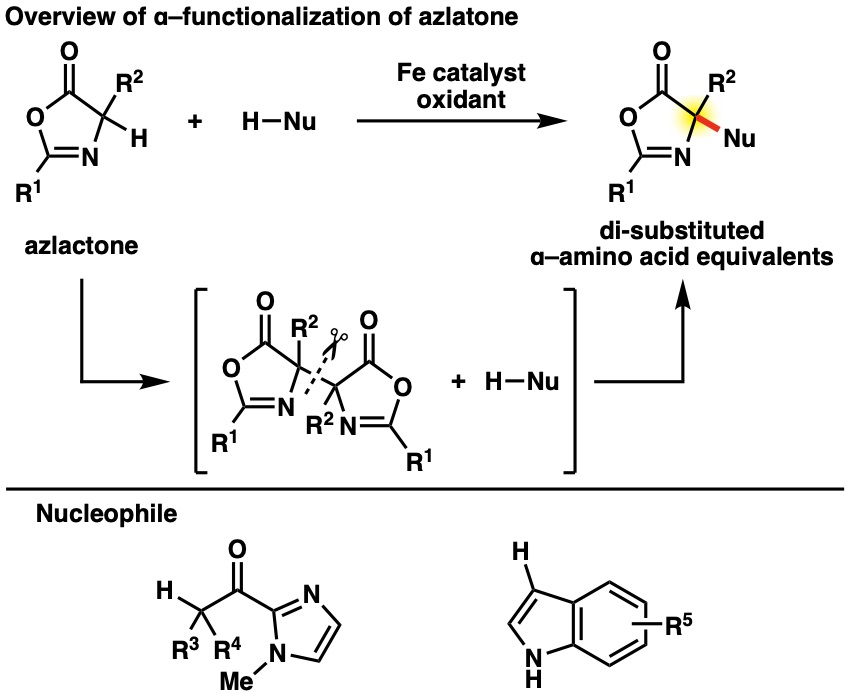
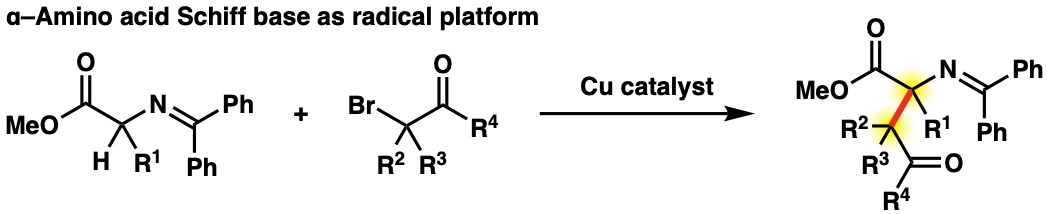
However, the azlactone method leaves room for improvement in environmental compatibility and substrate scope because of the multi-step process necessary in azlactone preparation and transformation. In recent years, we have been developing a cross-coupling reaction using α–amino acid Schiff base as a radical platform. This reaction enables the synthesis of unnatural α,β–bis-tetrasubstituted–α–amino acids, which were previously difficult to synthesize because of steric hindrance.
<Related Papers>
1. Takafumi Tanaka, Tsukushi Tanaka, Taro Tsuji, Ryo Yazaki, Takashi Ohshima
Org. Lett. 2018, 20, 3541–3544. DOI: 10.1021/acs.orglett.8b01313
2. Taro Tsuji, Takafumi Tanaka, Tsukushi Tanaka, Ryo Yazaki, Takashi Ohshima
Org. Lett. 2020, 22, 4164–4170. DOI: 10.1021/acs.orglett.0c01248
3. Yohei Matsumoto, Jun Sawamura, Yumi Murata, Takashi Nishikata, Ryo Yazaki, Takashi Ohshima
J. Am. Chem. Soc. 2020, 142, 18, 8498–8505. DOI: 10.1021/jacs.0c02707
4. Tetsu Ikeda, Haruka Ochiishi, Mana Yoshida, Ryo Yazaki, Takashi Ohshima
Org. Lett. 2022, 24, 369−373. DOI: 10.1021/acs.orglett.1c04042
5. Taro Tsuji, Kayoko Hashiguchi, Mana Yoshida, Tetsu Ikeda, Yunosuke Koga, Yusaku Honda, Tsukushi Tanaka, Suyong Re, Kenji Mizuguchi, Daisuke Takahashi, Ryo Yazaki, Takashi Ohshima
Nat. Synth. 2022, 1, 304–312. DOI: 10.1038/s44160-022-00037-0
